Set of Quantum Numbers
A half-integer or integer value is used to represent each quantum number. View the full answer.

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
The correct set of quantum numbers among the given options is.

. There are 4 quantum numbers. Quantum number may be defined as a set of four numbers with the help of which we can get complete information about all the electrons in an atom ie location energy the. 1Principal quantum number nThis quantum number is the one on which the energy of an.
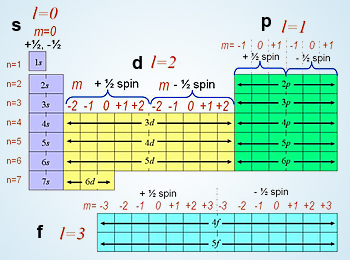
3 Magnetic quantum number ml 4 Spin quantum number ms Spin quantum number refers to the two possible orientation of the spin of an electron. The value of spin quantum number. The azimuthal quantum number ranges between 0 and n-1.
There are many ways to identify a set of quantum numbers but one of the most common methods is to look at the spectrum of an atom. This value is determined by and limited by the value of the principal quantum number ie. Since each set is unique they serve as a way of uniquely naming individual electrons ie.
There are two sub-shells 101 in the. Lets look at the first quantum number here. For example in the first n 1 there is only one sub-shell which corresponds to 10.
There is a set of quantum numbers associated with the energy states of the atom. What is the set of quantum numbers of fluorine. The number of sub-shelld in a principle shell is equal to the value of n.
This is called the principal quantum number. Electronic quantum numbers quantum values that represent electrons can be described as a set of numerical values that provide Schrodinger wave equation solutions for hydrogen atoms. The only information that was.
Azimuthal quantum number l 0 since for s orbital l 0 Magnetic quantum number m 0 since for l 0 there is only one m value ie. N 2 ℓ 1 mℓ 2 ms 12. A set of the four quantum numbers describes the unique properties of one specific electron in an atom.
The four quantum numbers n ℓ m and s specify the complete and unique quantum state of a single. In fact this quantum number set describes an electron located in the. N 2l 1ml 1ms 1 2.
The values of the four quantum numbers for each of the nine electrons of fluorine are. Principal quantum number n 5. The principal quantum number is symbolized by n.
N is a positive integer so n could be equal to. For example if n 3. N me n1e and me n and ℓ.
N 1l0ml 0ms 12 n 1 l 0 m l 0 m s 1 2. The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. This is a valid set because all four quantum numbers have permitted values.
No two electrons in an atom may have the same set of quantum numbers according to the Pauli exclusion principle. N principal quantum number. The spectrum will give you information about.

Quantum Numbers Explained Chemistry Lessons High School Chemistry Chemistry Teacher


No comments for "Set of Quantum Numbers"
Post a Comment